E-labeling and GS1 barcodes for patient safety: Traceability and access to drug information
Takao Orii1, Koichi Uemura2
1Tokyo Healthcare University Graduate School, Tokyo 113-8519, Japan
2General Manager, Solution Service Department I, GS1, Tokyo 113-8519, Japan
Corresponding author:
Takao Orii, E-mail: orii-tky@umin.ac.jp
For reprints contact: reprints@sppub.org
Received 15 March 2022; Accepted 02 June 2022; Available online 25 July 2022
INTRODUCTION
In 2019, Pharmaceutical and Medical Device Act was amended in Japan. Most of all pharmaceuticals sold in Japanese market have been marked an international barcode, GS1 barcode, on their packages, however, these barcoding is going to be mandatory from December 2022. And another measure, e-package insert (digitized package insert) and access to the information via GS1 barcodes, has been executed from August 2021. Under the policy, paper leaflet will be abolished and all digitized package inserts are collected on the database of PMDA, the Pharmaceuticals Medical Devices Agency, linked to the relevant international product identifier, Global Trade Item Number (GTIN), that is encoded in a GS1 barcode. The latest package insert information is going to be available at any time by URLs (Uniform Resource Locators) containing GTIN. An application for mobile devices such as smartphones has been developed, and you will be able to see the information by scanning GS1 barcodes on pharmaceutical packages.
HISTORY OF PHARMACEUTICAL GS1 BARCODING IN JAPAN
GS1 barcode marking started in 2006 with the announcement from the Ministry of Health, Labor and Welfare (MHLW), from high risk products such as blood product and injections. Since 2015, all products including blister packs have barcoded. From April 2021, secondary packages have to have a GS1 barcode encoding lot number and Expiration date in addition to GTIN.
In 2019, Japanese Pharmaceutical and Medical Device Act was amended. The Act is stronger force than the announcement of MHLW, and the barcoding on the pharmaceuticals is going to be mandate under the law from December 2022. (Figure 1)
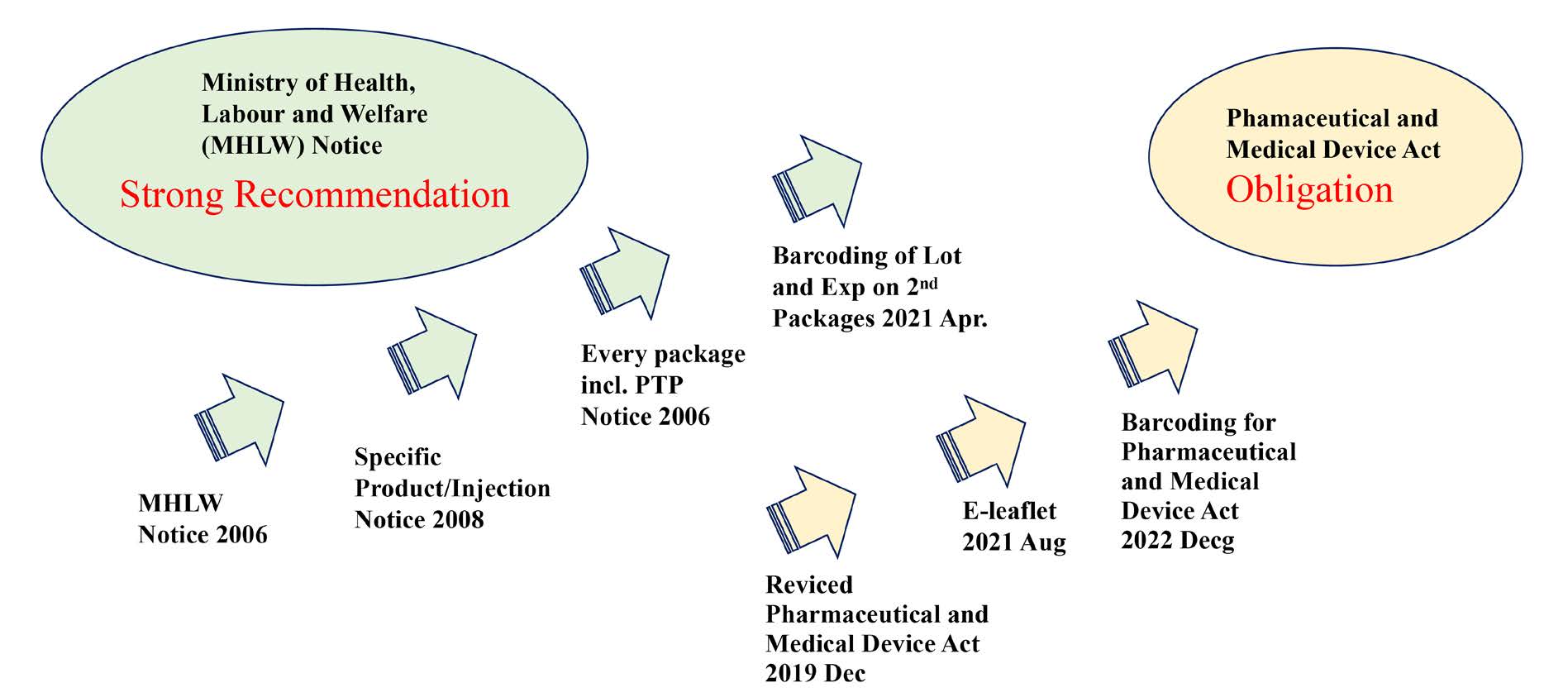
Figure 1: History of pharmaceutical GS1 barcoding in Japan
EXAMPLES OF GS1 BARCODES ON PHARMACEUTICAL PACKAGES IN JAPAN
All of pharmaceutical packages excluding Over-the-Counter (OTC), from primary such as ampules or blister packs to tertiary, have GS1 barcodes. Under the amendment law, more accurate barcodes marking is required, and active use of those barcodes in medical institutions should be expected. (Figure 2)

Figure 1: Barcode labeling on products
DIGITALIZATION OF PACKAGE INSERTS
From August 2021, all pharmaceutical’s package insert information has registered on website of PMDA with GTIN encoded to a GS1 barcode of every package. Healthcare providers can check the digitalized information with a specific App, Tenbun-Navi which was developed by GSI Japan, the Federation of Pharmaceutical Manufacturers’ Associations of JAPAN, and the Japan Federation of Medical Devices Association. (Figure 3,4)
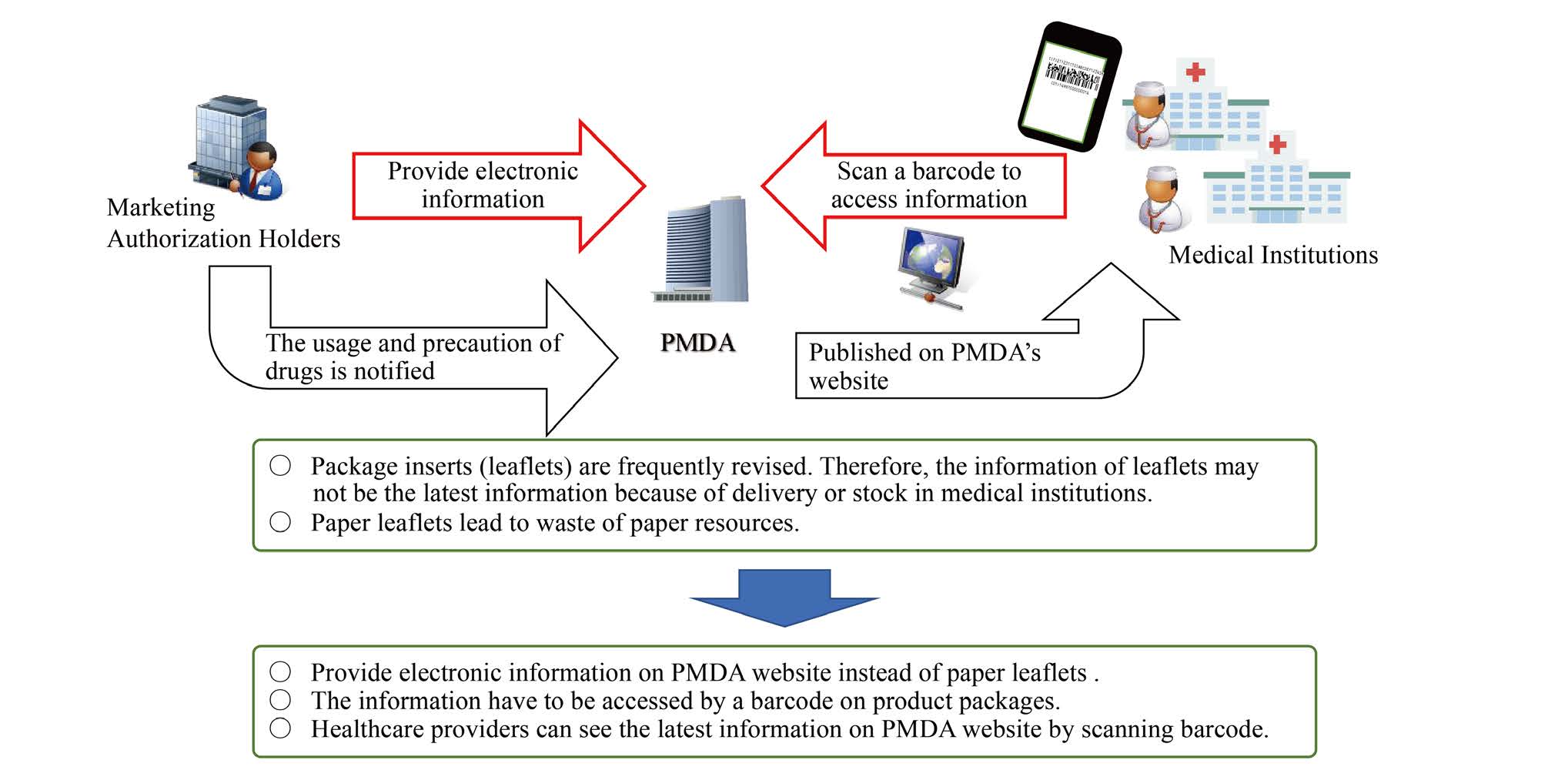
Figure 3: Digitalization of package inserts from August, 2021

Figure 4: Tenbun-navi. Specific App for access to e-llleaflets
DISCUSSION
For pharmaceuticals, GS1 barcodes are almost entirely displayed on every package in Japan. The barcodes can be used for many purposes and have provided a lot of benefits for healthcare providers. Especially the strategy in the digitalization of package inserts, GS1 barcode is expected to contribute to medical safety in respect of delivering a latest drug information, as well as providing identification of the drug itself.
We are now accumulating the result for efficiency of the digitalization of package inserts in our hospitals. We have not done detailed survey, however, the voice from pharmacists are quite well so far and there are no confusion. However we should keep taking notice for the situation because the paper inserts are going to be abolished gradually by the end of July 2023. From December 2022, GS1 barcodes for traceability will be mandatary displayed on the packages including medical devices. How to use these barcodes in the healthcare institution and how to link or share the data for traceability nationwide is the next challenge.
Source of Funding
None declared.
Conflict of Interest
None declared.
Declaration
The contents of this paper were presented at a conference in Dubai International Pharmaceutical & Technology Conference & Exhibition February 22–24, 2022. Dubal United Arab Emirates.